What is a Battery?
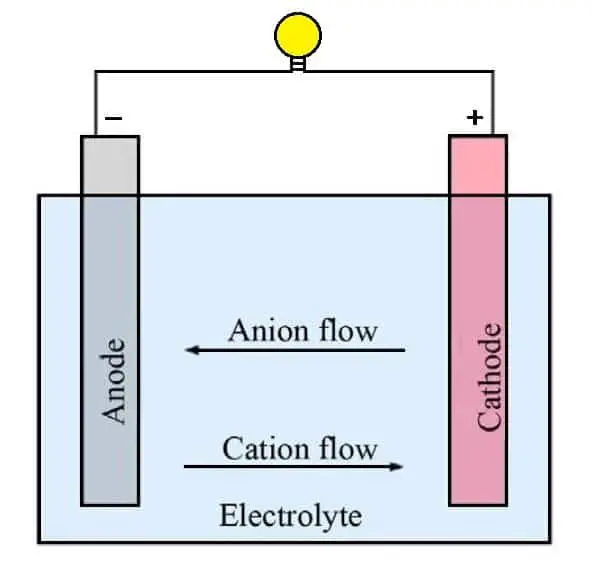
The electrochemical reaction in a battery is carried out by moving electrons from one material to another (called electrodes) using an electric current. The first battery was invented in 1800 by Italian physicist Alessandro Volta.
Whether you are an engineer or not, you must have seen at least two different types of batteries that is small batteries and larger batteries. Smaller batteries are used in devices such as watches, alarms, or smoke detectors, while applications such as cars, trucks, or motorcycles, use relatively large rechargeable batteries.
Batteries have become a significant source of energy over the past decade. Moreover, batteries are available in different types and sizes as per their applications. So we will discuss different types of batteries and their uses, so let’s get started.
How Does A Battery Work?
Classification of Batteries
- Primary battery
- Secondary battery
#1 Primary Battery
A primary battery is a simple and convenient source of electricity for many portable electronic devices such as lights, cameras, watches, toys, radios, etc. These types of batteries cannot be recharged once they are exhausted. They are composed of electrochemical cells whose electrochemical reactions cannot be reversed.
Generally, primary batteries are relatively inexpensive, lightweight, and convenient to use, with little or no maintenance. Primary batteries exist in many sizes and forms, ranging from coin cells to AA batteries. These are commonly seen in applications like pacemakers, animal trackers, wristwatches, remote controls, children’s toys, etc.
#2 Secondary Battery
Secondary batteries use electrochemical cells whose chemical reactions can be reversed by applying a certain voltage to the battery. It is also known as a rechargeable battery because it can be recharged after the battery’s energy is depleted. They are used as inverters for power supply as well as standalone power sources.
They are also used where it would be too expensive or impractical to use a single charged battery. Small-capacity secondary batteries are used in portable devices such as mobile phones, while heavy-duty batteries are found in electric vehicles and other high-drain applications.






Social Plugin